The mystery of the UDI medical products traceability code - what you need to know and how to solve it
On May 26, 2021, the guidelines for Unique Device Identification (UDI) came into force. After this date, numerous medical technology products must be marked with a barcode with an individual product identification barcode. If desired, the information contained in the barcode can be stored in an EU-wide database, Eudamed - a feature that will be of increasing importance in the future.
It is a critical topic—and a complex one because deadlines and responsible authorities vary, and you have to grapple with sometimes incomprehensible technical terms. MDR, GTIN, and ICCBBA? Read our article to find out what you need to know about UDI.
What is A UDI, and what is it for?
The UDI is a legal requirement. It stipulates that medical products and in-vitro diagnostic medical devices must be clearly marked with machine-readable labels worldwide. This provides several advantages:
Identification
Products are easily identifiable
Overview
The location of the devices can be tracked more easily
Safety
Detection of copies or counterfeits
Clarity
Increased patient safety with higher transparency
Transparency
Traceability of medical devices to improve market surveillance
To achieve this, each product is given a product identification—a Unique Device Identification, or UDI. This consists—similar to the EAN or NVE—of two parts: a company number and a self-assigned product identification.
In a product database provided by the EU (Eudamed), further product properties are available, for example, manufacturer, distributor, storage conditions, information on sterility, or reusability.
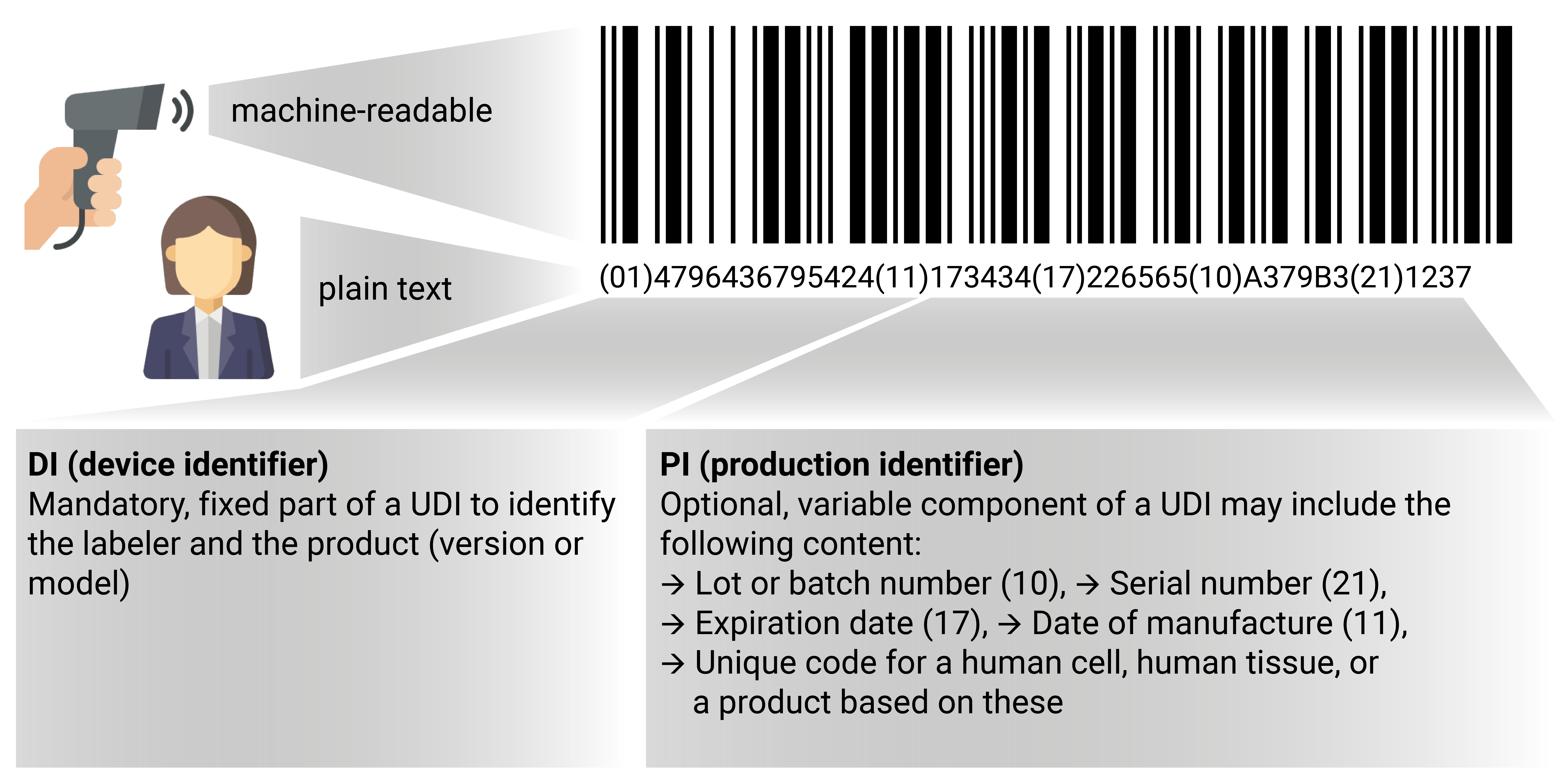
Part of the information is also additionally stored on the product label in the form of a barcode and additional text.
How is the UDI structured?
GS1-128 LINEAR BARCODE (commonly used to capture UDI)
GS1 DATAMATRIX CODE (often used to capture UDI)

The UDI barcode is built in a common, long-established standard format with defined identifiers provided by GS1 - similar to EAN and NVE specifications.
There are three UDI types:
A product group identifier or Basic UDI-DI that can be assigned to a group of products. Each individual product has its own number in the form of the UDI-DI. The Basic UDI-DI can be viewed in the Eudamed database, but is not printed on the product or packaging.
The product identifier or UDI Device Identifier (UDI-DI) corresponding to an item or type.
The production identifier or UDI Production Identifier (UDI-PI), which corresponds to each exact variation of a product, i.e., the specific individual product (serial number) or an entire lot.
The product, its unit of use, and the manufacturer can be identified using the product identifier, i.e., UDI-DI, This information must be printed directly on the product as well as on its packaging. Depending on the allocation body, it also contains the GTIN (Global Trade Item Number) (GS1), the UPN (Universal Product Number) (HIBCC), or the ISBT 128-PPIC (Processor Product Identification Code) (ICCBBA).
The UDI-DI is used in additional documents, such as the declaration of conformity, technical documentation, or Free Sales Certificates. The production identifier, UDI-PI, enables the identification of the individual serial numbers or batch of a product. Among other things, it contains the serial numbers, the lot or batch number, and expiration and manufacturing dates.
Who awards the UDI, and what is the medical device regulation (MDR)?
The UDI is allocated by specified bodies - which are defined in the MDR.
The MDR is the European Medical Device Regulation that came into force on May 25, 2017. After a transitional period of four years, its implementation has been mandatory since May 26, 2021.
Currently, there are the following official registration offices: GS1 with the GS1 code, HIBCC with the "Health Industry Barcode", ICCBBA with a UDI code, and IFA GmbH with the IFA code. While the first two are suitable as UDI codes for medical devices, the ICCBA code is used to identify products of human origin.
Which medical devices must be labled with a UDI?
Currently, it is mandatory to label all medical implants and class III medical devices in the EU with a UDI. This includes products with life-sustaining functions and thus a particularly high safety requirement. Gradually, the requirement will be extended to Class II (medium to increased risk) and Class I (low risk) medical devices.
From when do you need an UDI?
The UDI requirement for Class III medical devices and implants has been applied since May 26, 2021. The deadline for Class II is May 2023, with Class I following another two years later.
What are the UDI requirements?
Three UDI requirements apply across Europe:
1. BASIC UDI-DI
The basic UDI-DI serves as an identifier of a product group or family.
2. UDI DEVICE IDENTIFIER (UDI-DI)
The UDI-DI is a numeric or alphanumeric code that can be used to identify an item.
3. UDI PRODUCTION IDENTIFIER (UDI-PI)
The UDI-PI ensures traceability. To do this, it uses data such as the lot number, the expiry date, or the respective serial number.
UDI implementation deadlines in the USA
In the USA, the FDA (Food and Drug Administration) has also already made UDI mandatory. The Global Unique Identification Database (GUDID) serves as the collection point for the data.
How can the UDI be implemented with an ERP system, and what role does YAVEON play?
An ERP system can help companies meet the requirements imposed by UDI - because the necessary information is managed centrally in the system. A suitable ERP industry solution, such as YAVEON ProBatch, does this quickly and securely. An important step for the ongoing digitalization in medical technology.
YAVEON ProBatch extends the Microsoft Dynamics 365 Business Central ERP system with special features that are particularly relevant for the pharmaceutical, biotechnology, chemical, food, medical technology, and cosmetics industries.
For the UDI area, these are in detail:
Another advantage is that YAVEON has successfully supported clients in the regulated environment for many years and knows the challenges. Competent consulting, professional expertise, and a cooperative partnership bring projects to success - safely and sustainably.
The UDI is more than a rough guideline.
It is a legal requirement, compliance with which is not only binding, but also brings compelling benefits. In addition to transparency and assignment of drug types, patient safety is a top priority: Reliable traceability ensures that the composition, delivery, and use of medical technology products can be traced at all times. In the first step, the UDI was made mandatory for Class III medical devices in May 2021. Step by step, classes II and I are now being added. For many companies, this means a significant challenge.
Visit our website to find information expressly for the MedTech industry.
Author
Share article
Further interesting articles

Quality control for food
Quality control plays a particularly important role in the food industry. In this article, we explain what needs to be considered and what role software plays.








