Challenges that our ERP software solves for the biotechnology industry
Comply with regulations
Regulations and compliance requirements in the biotechnology industry are strict, and regular audits are the order of the day. Our ERP system lays the foundation to meet the stringent requirements in the regulated environment.
Withstand pressure to innovate
The competition never sleeps. Long development cycles, efficient production, and external funding add to your pressure to innovate. You can meet all these requirements more easily thanks to efficient processes with our ERP software for biotechnology.
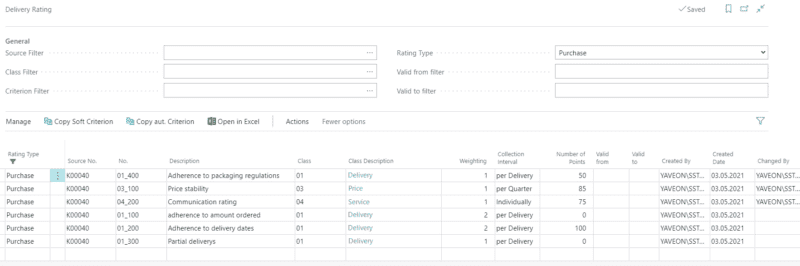
Batch overview
Traceability, responsiveness, and transparency in the handling of your products are essential factors, but difficult to achieve. With the batch management feature in our ERP software for biotechnology companies, you can keep an overview of the status.
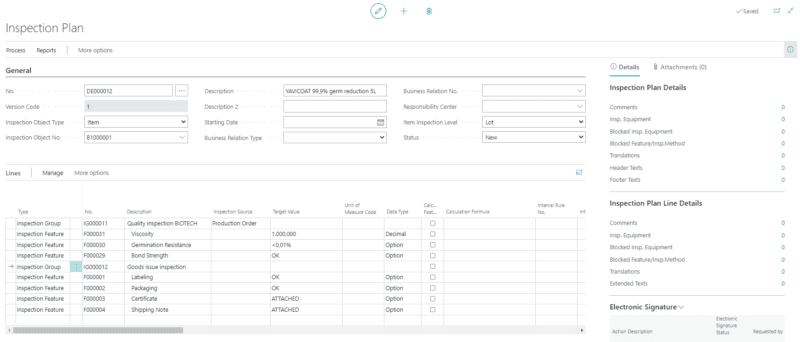
Strict quality controls
Your daily work is shaped by strict quality controls. With our ERP system for biotechnology companies, you can handle continuous quality inspection with the aid of digital support, and access the necessary information with one click.
Complex logistics
From the receipt of raw materials to the delivery of finished products, there are many stages in logistics and global supply chains in biotechnology. With our ERP, you can reduce complexity and exchange data digitally thanks to electronic data exchange.
Efficient production
Because time is a valuable commodity in production, you want to make the best use of it. How? With our ERP software, you can automate numerous processes, reduce the error rate, and increase efficiency of production processes.
A reference says more than a thousand words

Dyphox
„Of course we looked at other solutions. However, YAVEON ProBatch for the biotechnology industry was clearly more specific than the other systems. Moreover, the YAVEON board of directors was genuinely interested in us and we felt that we were taken very seriously."Klemens Wressnig Read more
Advantages of our ERP System for Biotechnology
Research and development, including recipe management
Do you want more time to focus on new product development? Our central and complete documentation makes it possible. This will please your customers and keep you one step ahead of the competition.
- Development & administration, as well as version management of your recipes/bill of materials for innovative products
- Targeted production release of recipes and recipe management
- Calculate the costs of new products directly in the system
- Include all relevant product features
- Selectively release recipes for manufacturing
- Preparation and shipment of samples
Conclusion
Do you need help to cope with the pressure to innovate while continuing to manufacture at a profit? The R&D functions of our ERP solution for biotechnology accomplish just that.
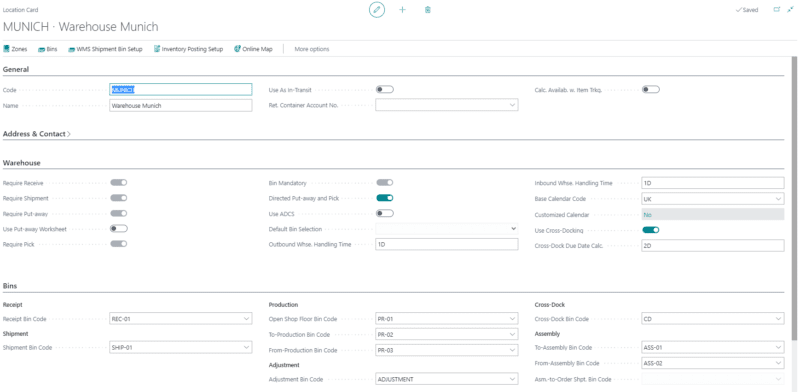
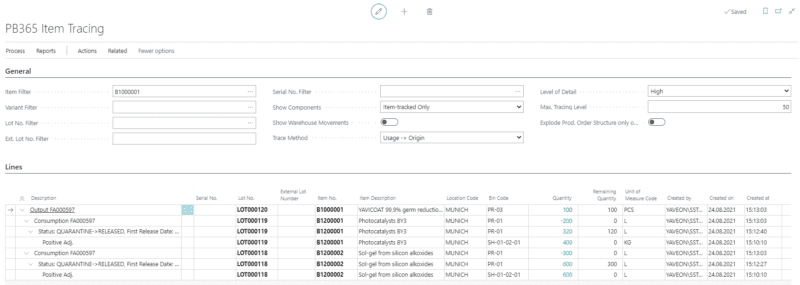
This is what our ERP YAVEON ProBatch for biotechnology looks like
This is what our ERP YAVEON Probatch for biotechnology looks like
FAQs: Answers to the most important questions about our biotech erp
You have questions? We have the answers.
Contact us now.
"Interested in products, questions, or any other concerns? Please feel free to contact us through our contact form. We will process your request as quickly as possible.